Catch the ChemBio SCENT! Screening, Collecting and Exploiting Novel Technologies
Updated 17 December 2021
1. Introduction
This Defence and Security Accelerator (DASA) competition is seeking proposals that can address challenges associated with screening for, the preservation of sample / evidence, and the attribution of hazardous chemical and biological (ChemBio) materials. New technology options or technical approaches could ultimately help both the successful identification of material and directly / indirectly aid in bringing perpetrators to justice. As well as addressing this diverse set of technical requirements, one of the goals of ‘Catch the ChemBio SCENT!’ is to engage with a non-traditional ChemBio supplier base (i.e. suppliers who have not worked in Defence and Security before) in order to yield innovative solutions. Up to £1M is available to fund multiple emerging innovations (low technology readiness level (TRL)) to demonstrate proof-of-concept during Phase 1 of the competition. It is expected that proposals will not exceed £100K. It is anticipated that there will be a second phase of this competition with additional funding, which will seek to further develop promising solutions from Phase 1 as well as identify and accelerate other solutions at a higher TRL. This competition is jointly funded by the UK Ministry of Defence (MOD) and the US Department of Defense (DoD), the relationship will operate under and be governed by an extant memorandum of understanding between both nations. Both the MOD and DoD will have access to proposals submitted under this competition in order to jointly assess which proposals to fund.
2. Background
The production, stockpiling or use of hazardous chemical and biological (ChemBio) materials for nefarious purposes represents an enduring and often high profile issue for both the Defence and Security agencies. Activities of this nature would be considered a breach of either the Chemical Weapons Convention or the Biological and Toxin Weapons Convention. Investigation into events involving ChemBio materials have many technical challenges; starting from the initial point of collection by the attending first responder, all the way through to the screening and analytical tools used to identify and / or potentially attribute the material of interest. ‘Catch the ChemBio SCENT!’ is a competition that is being joint funded by the UK Ministry of Defence and US Department of Defense. The competition is being administered by the Defence and Security Accelerator (DASA).
‘Catch the ChemBio SCENT!’ seeks novel and innovative approaches in order to address challenges associated with sample collection, rapid screening, the preservation of sample / evidence during storage or transportation and potential forensic exploitation to aid attribution of ChemBio materials (Table 1). Ultimately, effective new approaches could help either a successful identification of ChemBio materials and / or bring perpetrators to justice.
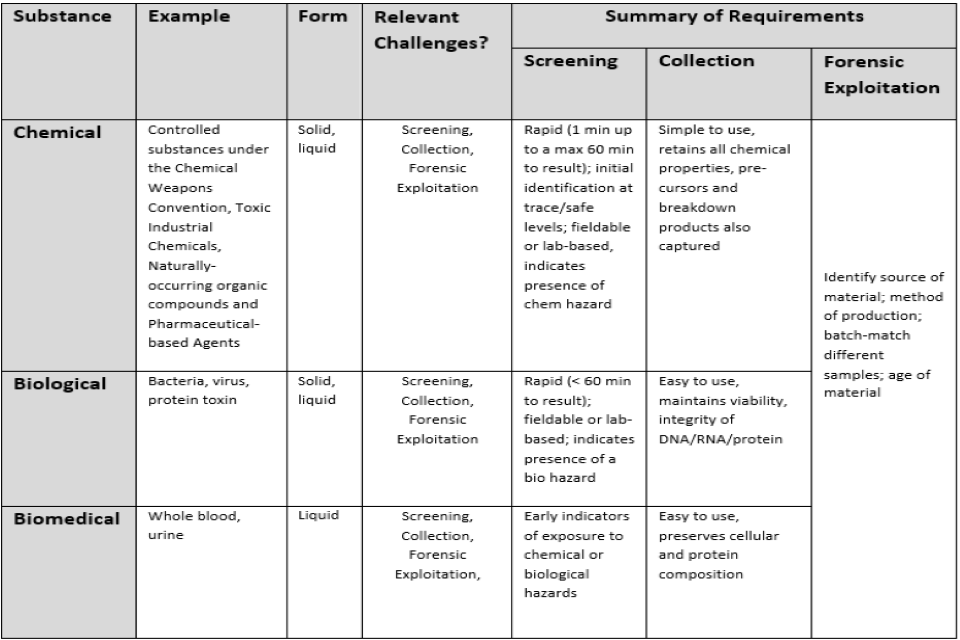
Table 1. Summary of the technical requirements that for the basis of the “Catch the ChemBio Scent!” Competition. Novel technologies are being sought that can address the screening, collection and forensic exploitation requirements outlined.
Screening for hazardous materials represents a key early step required during a ChemBio incident (i.e. in order to ‘Catch the ChemBio SCENT!’). Screening technologies are a critical tool for the analysis of unknown samples both in the field (i.e. deployed on Operations) and in a highly controlled environment (i.e. within an analytical laboratory); providing both a safety (i.e. triage) function and potential initial indication as to the hazardous material that is present. Therefore, it is vital that the identification of hazardous ChemBio occurs correctly at this stage in order to ensure that handling and manipulation of the material is undertaken in a safe and controlled manner. Therefore, technology options that can rapidly (i.e. ideally within minutes but up to a maximum of 60 minutes) and correctly identify agent classes and the nature / properties / characteristics of the hazard will be key to increasing the safety and the speed of analysis (Table 1).
The sampling of hazardous materials needs to be a low burden, technically simple activity which can be undertaken by a diverse range of operators, from first responders, to military personnel and / or scientists. Moreover, the samples may need to be recovered from a broad range of contexts, including from within austere environments with potentially extreme climatic conditions through to urban populated areas. This poses a number of challenges including stabilising the material from degradation in transit to the laboratory for analysis. Crucially, the quality of the sample submitted to the laboratory directly dictates the level of analysis that can be undertaken on that material and, in turn, the quality of the resulting analytical data that can be produced. Therefore techniques that maintain the physical integrity and stability (i.e. chemical) or viability (i.e. biological) for the duration of transport are key to a successful analysis and attribution capability. Parallels may be found, from a Science and Technology perspective, in the medical and / or biomedical device sector (e.g. stabilisation of blood products and / or stem cells).
Finally, attribution activities help to bridge the gap between the laboratory and traditional forensic approaches by providing information on the provenance of the ChemBio material. Principally of interest here is the ability to identify the agent present (e.g. based on breakdown products), how the material was made / produced / stored (e.g. based on impurities, trace or background contaminants) and potentially linking materials derived from different sources (i.e. from release site of a terrorist event to an improvised laboratory). Parallels, from a technical perspective, may be found here in approaches used by seemingly unrelated sectors (e.g. in the authentication of high value food / drink or in materials used in the aerospace industry, or in investigations into the provenance of valuable art).
Recent examples of sampling and analysis undertaken in this area include:
-
Samples collected during Operation Saddleback
-
OPCW sampling and analysis of CW by Daesh in Iraq
-
OPCW sampling and analysis of mustard produced and used by Daesh in Iraq
3. Competition Scope
The challenges outlined in this competition are diverse in nature, therefore it is anticipated that proposals will address either the screening, collection or forensic exploitation challenge (i.e. rather than covering multiple requirements).
Key current limitations of applying screening, collection and forensic exploitation technologies to these challenges include:
-
collection technologies can sample materials from surfaces but do not protect and stabilise the sampled materials for storage and transport (i.e. without cold chain support that are often unavailable in austere environments or in a non-permissive operational context)
-
screening technologies are predominantly focused on identifying specific (known) or well-characterised ChemBio Hazards. Screening for a wider range of materials is restricted due to the limitations of handheld or field based screening technologies being able to detect a broad range of undefined compounds. The ability to detect a broader range of chemicals requires significant laboratory equipment / expertise / time
-
forensic exploitation technologies are in their infancy in the field of attribution and often require data intensive approaches taking weeks or months to undertake. New ways of working that expedite the process and / or identify new signatures to enable the source material to be attributed across the timeframes traditionally associated with analysis (i.e. hours, days) are required.
We are seeking novel and innovative technologies and approaches that will overcome these limitations and enhance the range of tools available when undertaking an investigation following an overseas or Homeland ChemBio event. We expect that these tools will ultimately enhance the capabilities of the International Community to contend with many technical challenges associated with sample screening, collection (including storage and transportation) and forensic exploitation for attribution purposes following a ChemBio event. Promising technology options identified here will improve the efficiency, efficacy and likelihood of success of an investigation into ChemBio incident.
We are looking for innovations that will form the basis of the next generation of screening, collection and forensic exploitation approaches that will be utilised during the investigation of a CB event. Fundamentally, your proposed solution must improve an element of the investigative system into hazardous ChemBio materials but must not increase the risks to either the Operator, the wider population or the handling and containment of hazardous ChemBio materials within a laboratory. We are also interested in proposals that develop or repurpose existing technologies used in other sectors.
4. Competition Challenges
The challenges outlined in this competition are diverse in nature, therefore it is anticipated that proposals will address either the screening, collection or forensic exploitation challenge (i.e. rather than covering multiple requirements). Typical examples of hazardous materials that are required to be analysed are Bio (e.g. bacteria, virus, protein toxin) and Chem (e.g. materials covered by the Chemical Weapons Convention, Toxic Industrial Chemicals, and pharmaceutical based agents). The ChemBio materials can also be submitted in different forms including environmental (e.g. soil, vegetation) or biomedical (e.g. whole blood, urine) in composition. There are three main challenge areas where innovative solutions and approaches are being sought:
4.1 Challenge 1: New screening technologies for rapidly detecting or identifying the presence or class of hazardous ChemBio materials in a sample
The principal goal for this challenge is to enable the rapid classification of ‘unknown’ hazardous substances in the case of not being able to detect or identify a specific ChemBio agent. In essence, these technologies will aid in the initial screening and triage of whether that is deployed on scene during sample collection or upon submission to the laboratory for analysis (i.e. a key safety feature of any analytical laboratory). Crucially, in order to achieve this later requirement, technologies should screen for the presence of Chemical and / or Biological materials down to the lowest commercially available instrument sensitivity levels (e.g. nanograms, parts per million). Innovative technologies that aid the screening and / or triage of samples rapidly upon collection have the capacity to provide vital information ahead of more in-depth processing and will be key to increasing the safety and speed of the overall timeline for analysis.
We are looking for techniques or approaches that can achieve at least some of the elements of the following:
-
Provide additional information on either the physical / biological / chemical properties of the ChemBio material aiding classification or identification
-
Either a single, a combination of distinct orthogonal approaches, or an adjunct to existing commercial-off-the-shelf technology
-
Identification of target analytes (or biomarkers of exposure) through the analysis of environmental or biomedical samples
-
Work rapidly (i.e. provide a result in minutes and up to a maximum of 60 minutes)
There are also two principle concepts of use for the approaches of interest here:
-
Fieldable techniques, operable by non-specialist users (deployed systems should be fast, light, mobile, ruggedised and simple to use)
-
Laboratory-based techniques, operated by Suitably Qualified Experienced Personal (SQEP)
4.2 Challenge 2: New sampling collection systems that better maintain the integrity and viability of hazardous samples
The goal of this challenge is to retrieve samples from an Operational setting (Overseas or Homeland) using a system(s) that preserves the properties of the materials collected.
Solutions should fulfil at least some of the following criteria:
-
Include a device that safely and appropriately contains and stores hazardous chemical or biological materials that can be transported easily back to the laboratory.
-
Be immediately self-stabilising for carriage upon sampling and / or are a ‘collection system’ (i.e. materials / solutions that can be added / mixed with a sample in order to maintain integrity during transport)
-
Technology should be compatible with chemical and / or biological material
-
Approaches that support biomedical and / or clinically-relevant samples are also of interest (e.g. maintaining the cellular composition / viability, genetic, proteomic material)
-
Easy to use (i.e. can be put in the hands of collectors with minimal specialist training)
-
Can be operated easily within PPE (e.g. chemical resistant butyl gloves, respirator)
-
Once fully developed the technology must be safe for transportation via air, sea and land
-
Must stabilise materials for a defined timeframe (i.e. minimum 14 days; stretch target = 90 days)
-
Defined environmental conditions for stability studies (i.e. basic condition = ambient room temperature; stretch target = up to 50°C)
-
Materials to be stabilised without any form of cold chain storage requirement
-
Defined area of collection for performance assessment (i.e. Classic swabbing = 10 cm2) Defined high efficiency collection (e.g. minimum acceptance might be greater than 30 – 50 % recovery from an agreed standardised surface; stretch target = greater than 90 %). Examples of typical surfaces to be sampled from include:
-
Hard / smooth / nonporous surfaces
-
Soft / rough / porous substrates
-
Skin (exposed and / or protected)
-
Clothing and personal protective equipment
-
4.3 Challenge 3: New analytical approaches to improve the forensic exploitation and the attribution of ChemBio materials and / or recovered chemical / biological weapons material
The goal of this challenge is to maximise the information and breadth of approaches that can be used to analyse a sample enabling its underlying properties to be determined. Acquiring this level of detail about a sample increased the opportunity for the perpetrators of the use of ChemBio materials to be identified during an investigation.
We are looking for techniques, technologies or approaches that enable attribution by:
-
Being able to determine the original or source (i.e. provenance)
-
Providing information on how the target of interest or agent was produced
-
How it was stored and / or who made the material
-
Determining the target of interest or agent used based on other related analytes detected in the sample
-
Facilitating the potential for ‘batch matching’ (e.g. linking samples collected from different sites or sources).
5. Clarification of what we want
We want novel ideas to benefit users working in UK and US Defence and Security. Your proposal should include evidence of:
-
Theoretical development, methodological advancement or proof of concept research which can demonstrate potential for translation to practical demonstration in later phases
-
Innovation or a creative approach
-
Clear demonstration of how the proposed work applies to any defence and security context
-
Consideration of how a different industry might address this problem (i.e. application of existing techniques in the new context of investigating a ChemBio event)
-
Proposals that address at least one of the specified challenges (i.e. proposals do not need to address all three) for either Chem, Bio and / or Biomedical materials
-
A robust supply chain for the key elements or aspects of the proposal to ensure there is enduring availability
-
That the approach is robust enough that it can be feasibly technology transferred into other analytical laboratories if required
-
Have the potential to be fully validated and accredited to an appropriate quality (i.e. ISO/IEC IS017025:2017)
6. Clarification of what we don’t want
For this competition we are not interested in proposals that:
-
Constitute consultancy, paper-based studies or literature reviews which just summarise the existing literature without any view of future innovation
-
Are an identical resubmission of a previous bid to DASA or MOD without modification
-
Offer demonstrations of commercial off-the-shelf (COTS) products requiring no experimental development (unless applied in a novel way to the challenge)
-
Offer no real long-term prospect of integration into defence and security capabilities
-
Offer no real prospect of out-competing existing technological solutions
-
Are unaffordable (for example a single-use sacrificial system which is cost-prohibitive)
-
Cannot demonstrate feasibility within the timescale of the phase of the competition
-
Offer only a written report - we are looking for a demonstration
-
Are completely unproven ideas / concepts (i.e. TRL1)
-
Are for the procurement of standard pieces of analytical equipment from a commercial supplier or third party distributor
-
Are for the procurement of any form of commercial off-the-shelf (COTS) product or service without any form of testing or planned testing to support their application for one of the challenges outlined above.
7. Exploitation
It is important that over the lifetime of DASA competitions, ideas are matured and accelerated towards appropriate end users to enhance capability. How long this takes will be dependent on the nature and starting point of the innovation. Early identification and appropriate engagement with potential end users during the competition and subsequent phases are essential in order to develop and implement an exploitation plan.
All proposals to DASA should articulate the expected development in technology maturity of the potential solution over the lifetime of the contract and how this relates to improved operational capability against the current known (or presumed) baseline. Your deliverables should be designed to evidence these aspects with the aim of making it as easy as possible for potential collaborators / stakeholders to identify the innovative elements of your proposal in order to consider routes for exploitation.
A higher technology maturity will be expected in any potential subsequent phases. You may wish to include some of the following information, where known, to help the assessors understand your exploitation plans to date:
-
the intended defence or security users of your final product and whether you have previously engaged with them, their procurement arm or their research and development arm
-
awareness of, and alignment to, any existing end user procurement programmes
-
the anticipated benefits (for example, in cost, time, improved capability) that your solution will provide to the user
-
whether it is likely to be a standalone product or integrated with other technologies or platforms
-
expected additional work required beyond the end of the contract to develop an operationally deployable commercial product (for example, ‘scaling up’ for manufacture, cyber security, integration with existing technologies, environmental operating conditions)
-
additional future applications and wider markets for exploitation
-
wider collaborations and networks you have already developed or any additional relationships you see as a requirement to support exploitation
-
how your product could be tested in a representative environment in later phases
-
any specific legal, ethical, commercial or regulatory considerations for exploitation
Longer term studies may not be able to articulate exploitation in great detail, but it should always be clear that there is some credible advantage to be gained from the technology development.
This competition is being carried out as part of a wider UK MOD programme and with cognisance of cross-Government initiatives. Ultimately this research, if successful, has a clear and obvious exploitation and potential integration within the existing UK and US Operational Capability. In addition, given the wide range of stakeholders that require support during an investigation into a ChemBio event there are clearly opportunities to exploit any successful research developments and / or tools across Defence, Homeland Security and with International Organisations. Finally, given the global nature of the research challenges being presented, we are undertaking this competition jointly with US DoD, therefore there are clear partnering opportunities with appropriate agencies within the US.
8. How to apply
Proposals for funding to meet these challenges must be submitted by 17th January 2022 at midday (1200 Greenwich Meantime; GMT) via the DASA submission service for which you will be required to register.
The total funding available for Phase 1 of this competition is up to £1M (ex VAT), but individual proposals cannot exceed £100K (ex VAT). If successful, contracts will be awarded for a maximum duration of 12 months.
Any further phases will be open to applications from all suppliers and not just those that submitted Phase 1 successful bids. Proposals in Phase 2 will potentially be for longer contract durations and a commensurate higher level of funding in comparison to the current Phase 1 call for proposals.
Further guidance on submitting a proposal is available on the DASA website.
9. What your proposal must include
The proposal should focus on the Phase 1 requirements but must also include a brief (uncosted) outline of the next stages of work required for exploitation.
When submitting a proposal, you must complete all sections of the online form, including an appropriate level of technical information to allow assessment of the bid and a completed finances section. Completed proposals must comply with the financial rules set for this competition. The upper-limit for this competition is £100K (ex VAT). Proposals will be rejected if the financial cost exceeds this capped level. You must include a list of other current or recent government funding you may have received in this area if appropriate, making it clear how this proposal differs from this work.
A project plan with clear milestones and deliverables must be provided. Deliverables must be well defined and designed to provide evidence of progress against the project plan and the end-point for this phase; they must include a final report. You should also plan for attendance at a kick-off meeting at the start of the contract, monthly project review meetings with your Technical Partner and the Project Team (either virtual or in person at your facility) as well as providing monthly progress reports (a report template will be provided for this purpose). All will be in the UK by default unless mutually pre-agreed with the supplier and / or if an onsite supplier assurance meeting is required. Your proposal must demonstrate how you will complete all activities / services and provide all deliverables within the competition timescales (12 months maximum). Proposals with any deliverables (including final report) outside the competition timeline will be rejected as non-compliant. A face-face end of project event will take place in the UK at the end of Phase 1, at which all suppliers will be expected to attend and present their key results to Stakeholders from across the International Defence and Security community.
A resourcing plan must also be provided that identifies the nationalities of those proposed research workers that you intend working on this phase. In the event of proposals being recommended for funding, the DASA reserves the right to undertake due diligence checks including the clearance of proposed research workers. Please note that this process will take as long as necessary and could take up to 6 weeks in some cases for non-UK nationals.
You must identify any ethical / legal / regulatory factors within your proposal and how the associated risks will be managed, including break points in the project if approvals are not received. MODREC approvals can take up to 5 months therefore you should plan your work programme accordingly. If you are unsure if your proposal will need to apply for MODREC approval, then please contact DASA for further guidance.
Requirements for access to Government Furnished Assets (GFA), for example, information, equipment, materials and facilities, should be included in your proposal. DASA cannot guarantee that GFA will be available.
Failure to provide any of the above listed will automatically render your proposal non-compliant.
10. Cyber risk assessment
DASA has completed a Cyber Risk Assessment (CRA) for this competition. In order to submit to this competition, innovators are required to work towards cyber resilience. If selected for funding, the innovator must prove cyber resilience before a contract will be awarded.
Innovators must complete a Supplier Assurance Questionnaire (SAQ) here, using the DASA Risk Assessment Reference (RAR) for this competition: RAR-948719413 and answer questions for risk level ‘Low’.
The Defence Cyber Protection Partnership (DCPP) will review your SAQ submission and respond with a reference number within 2 working days. The completed SAQ form and resulting email response from DCPP must be downloaded and included within the DASA submission service portal when the proposal is submitted. Please allow enough time to receive the SAQ reference number prior to competition close at midday on 17th January 2021.
If the proposal is selected for funding, the SAQ will be evaluated against the CRA for the competition, and it will be put it into one of the following categories:
-
Compliant – no further action
-
Not compliant – if successful in competition and being funded, the innovator will be required to complete a Cyber Implementation Plan (CIP) before the contract is placed, which will need to be reviewed and agreed with the relevant project manager
Innovators can enter a proposal without all controls in place, but are expected to have all the cyber protection measures necessary to fulfil the requirements of the contract in place at the time of contract award, or have an agreed Cyber Implementation Plan (CIP). The CIP provides evidence as to how and when potential innovators will achieve compliance. Provided the measures proposed in the Cyber Implementation Plan do not pose an unacceptable risk to the MOD, a submission with a Cyber Implementation Plan will be considered alongside those who can achieve the controls.
A final check will be made to ensure cyber resilience before the contract is placed. Commercial staff cannot progress the competition / procurement without it. This process does not replace any contract specific security requirements.
Further guidance for completing this process can be requested by emailing accelerator@dstl.gov.uk.
Additional information about cyber security can be found at: DCPP: Cyber Security Model industry buyer and supplier guide.
11. Export control
The outputs / deliverables from contracts awarded as a result of this competition will be shared under an extant memorandum of understanding between the UK MOD and US DoD. This will facilitate the unimpeded exchange of proposals, prototypes and associated information between the UK and US governments. However, this effective exemption from export controls only applies to the UK and US, not to third countries, and all bidders must therefore abide by the export control requirements of their originator country. All relevant export control regulations will apply if a company ultimately wants to sell a developed solution to a foreign entity. All bidders must ensure that they can obtain, if required, the necessary export licences for their proposals and developments, such that they can be supplied to the UK and US. Additionally, if we believe that you will not be able to obtain export clearance, additional checks may be conducted, which may also result in your proposal being sifted out of the competition.
Specific to US applicants: US bidders must obtain the proper requisite export licence before submitting technical information to the DASA. In addition, bidders are strongly encouraged to review relevant export control sections of the International Traffic in Arms Regulations (ITAR), to ascertain if any sections pertain to the requested activity, noting that recent amendments expedite processing of licences for export to the UK. Pertinent information should be referenced in the applicant’s transmittal letter and in Block 20 (Purpose) of the permanent export licence form (DSP-5) before submitting application to the Department of State’s Directorate of Defense Trade Controls (DDTC). The bidder may wish to review the guidelines for an export licence request prior to submission. This information can be found on the DDTC website.
12. Public facing information
When submitting your proposal, you will be required to include a proposal title and a short abstract. The title and abstract you provide will be used by DASA, other UK government departments and the US Department of Defense, to describe the project and its intended outcomes and benefits. It will be used for inclusion at DASA events in relation to this competition and included in documentation such as brochures. The proposal title will also be published in the DASA transparency data on GOV.UK, along with your company name, the amount of funding, and the start and end dates of your contract.
13. Supplier Collaboration
We encourage collaboration between organisations for this competition. To support this we have a short survey to collect details of those who wish to explore collaboration possibilities. If you are interested in a collaboration, please complete the survey and your details will be circulated among other potential suppliers who have completed the survey and are interested in collaborating.
If you choose to complete the Supplier collaboration survey, please be aware that all of the information you submit in the survey will be provided to other Suppliers who also complete the survey.
14. How your proposal will be assessed
At Stage 1, all proposals will be checked for compliance with the competition document and may be rejected before full assessment if they do not comply. Only those proposals who demonstrate their compliance against the competition scope and DASA mandatory criteria will be taken forward to full assessment. Failure to achieve full compliance against Stage 1 will render your proposal non-compliant and will not be considered any further:
| Mandatory Criteria | |
|---|---|
| The proposal outlines how it meets the scope of the competition | Within scope (Pass) / Out of scope (Fail) |
| The proposal fully explains in all three sections of the DASA submission service how it meets the DASA criteria | Pass / Fail |
| The proposal clearly details a financial plan, a project plan and a resourcing plan to complete the work proposed in Phase 1 | Pass / Fail |
| The proposal identifies the need (or not) for MODREC approval | Pass / Fail |
| The proposal identifies any GFA required for Phase 1 | Pass / Fail |
| Maximum value of proposal is £100k | Pass / Fail |
| The proposal demonstrates how all research and development activities / services (including delivery of the final report) will be completed within 12 months from award of contract (or less) | Pass / Fail |
| The bidder has obtained the authority to provide unqualified acceptance of the terms and conditions of the Contract. | Pass / Fail |
| The bidder has done all of the following: submitted a Supplier Assurance Questionnaire (SAQ) number; attached the email from DCPP; attached the submitted SAQ form | Pass / Fail |
Proposals that pass Stage 1 will then be assessed against the standard DASA assessment criteria (Desirability, Feasibility and Viability) by subject matter experts from the MOD (including Dstl), US DoD, other government departments and front-line military commands. You will not have the opportunity to comment on assessors comments.
DASA reserves the right to disclose on a confidential basis any information it receives from bidders during the procurement process (including information identified by the bidder as Commercially Sensitive Information in accordance with the provisions of this competition) to any third party engaged by DASA for the specific purpose of evaluating or assisting DASA in the evaluation of the bidder’s proposal. In providing such information the bidder consents to such disclosure. Appropriate confidentiality agreements will be put in place.
Further guidance on how your proposal is assessed is available on the DASA website.
After assessment, proposals will be discussed internally at a Decision Conference where, based on the assessments, budget and wider strategic considerations, a decision will be made on the proposals that are recommended for funding.
Proposals that are unsuccessful will receive feedback after the Decision Conference.
15. Things you should know about DASA contracts
Please read the DASA terms and conditions which contain important information for suppliers. For this competition we will be using the Innovation Standard Contract (ISC), links to the contract here: Terms and Schedules. We will require unqualified acceptance of the terms and conditions, therefore if applicable please ensure your commercial department have provided their acceptance.
Funded projects will be allocated a Project Manager (to run the project) and a Technical Partner, either from the UK, US or both, (to act as a technical point of contact), providing technical review of deliverables and an interface between the UK and US stakeholder communities. In addition, the DASA team will work with you to support delivery and exploitation including where appropriate introductions to end-users and business support to help SMEs develop their business.
DASA also collects information from projects after the project has concluded and you should expect to be contacted once your project has completed for measurement purposes.
We will use deliverables from DASA contracts in accordance with our rights detailed in the contract terms and conditions. This competition is jointly funded by the MOD and the US DoD, and MOD shall share all deliverables with, and provide rights of use to information therein to, US DoD under and in accordance with the terms of the Memorandum of Understanding.
For this phase, up to £1M is currently available to fund proposals. There may be occasions where additional funding from other funding lines may subsequently become available to allow us to revisit those proposals deemed suitable for funding but where limitations on funding at the time prevented contracts being awarded. In such situations, DASA reserves the right to keep such proposals in reserve. In the event that additional funding subsequently becomes available, DASA may ask whether you would still be prepared to undertake the work outlined in your proposal under the same terms.
16. Phase 1 Key Dates
-
Tuesday 16th November 2021 – Launch Webinar
-
Wednesday 17th & Thursday 18th November 2021 – Pre-bookable 1-to-1 telecom sessions
-
Monday 17 January 2022 (1200 Greenwich Meantime (GMT) – Competition close
-
Tuesday 22nd March 2022 - Feedback release
-
Monday 14th March 2022 – Contracting
17. Supporting events
-
Tuesday 16th November 2021 – Launch Webinar - A dial-in session providing further detail on the problem space and a chance to ask questions in an open forum. If you would like to participate, please register on the Eventbrite page.
-
Wednesday 17th & Thursday 18th November 2021 – 1-to-1 Q&A - A series of 15 minute 1-to-1 teleconference sessions, giving you the opportunity to ask specific questions. If you would like to participate, please register on the Eventbrite pages below.
18. Help
Competition queries including on process, application, commercial, technical and intellectual property aspects should be sent to accelerator@dstl.gov.uk, quoting the competition title. If you wish to be added to the campaign on ECS please email a request into the accelerator inbox, this will ensure you receive future updates on this competition.
While all reasonable efforts will be made to answer queries, DASA reserves the right to impose management controls if volumes of queries restrict fair access of information to all potential suppliers.
19. Launch Event Q&A
19.1 General questions
Q: Would a technology under development within a different DASA call, but which can be adapted for the screening of chemical materials, be eligible for funding?
A: Yes, as long as the proposals are distinctly different. Whether the other funding is from DASA or another body, use your proposal to highlight what is being proposed for this competition.
Q: Within the military, who do you envisage being the end-users? CBRN-dedicated staff, combat medics, etc?
A: The competition is about identification of ChemBio substances in the field and finding a way to screen for them in order to get an idea what is present. And if this can’t be done, we want to be able to collect a sample and it get back to the lab for more in-depth analysis. There are many military perspectives, many uses and many potential end-users.
Q: When is the funding for the challenge provided?
A: Successful bidders will be awarded a contract which will include a milestone payment plan provided as part of the bidder’s proposal. Please note that we cannot accept requests for advance payments and the final milestone needs to be 20% value of the total bid.
Q: The application requires a DUNS number. Is it possible for a PhD student funded by a university to have one of these?
A: Ask at your department at your university, they should be able to supply it or find out how to apply for one.
Q: If we design a prototype, how we can access samples for proof of concept?
A: We are not expecting suppliers to have the ability to handle ChemBio materials as this would immediately limit the numbers and types of supplier we are looking to engage through this activity. Proof of principle that an approach or technology can work for its intended purpose should be possible without specifically requiring hazardous substances. We hope there will be a phase 2 after this competition. Then we will be able to work with a partner / contractor more closely and support further work involving test and evaluation with high hazard materials.
Q: What is the limit of funding for a project?
A: We expect to fund 10-12 proposals with the funding available, up to approximately £100K per project, for a up to 12 months; see the competition document, section 8 How to Apply.
Q: Do the terms / conditions include a ‘liability’ clause where all the risk from deployment fail lies with inventors?
A: The contract terms and conditions include a ‘Limit of Contractor’s Liability Clause’ which assigns an aggregated value to each of five heads of risk. These include damage to Government Establishments, material breach, damage to Government Equipment, damage to the goods being purchased and any other risk which does not fall in the first four. However, bidders would not necessarily be held liable for deployment fail if they have followed their own proposal and have done what was required.
Q: How do you protect suppliers’ IP?
A: The contract for this competition includes the IP condition DEFCON 705. DEFCON 705 vests ownership of IP with the contractor, with MOD securing user rights. Thus, the IP is owned by the contractor, and consequently the innovator can use the IP it generates in the future. Our online portal protects IP and the individuals who have access to it are civil servants bound by the civil service code; they are not allowed to discuss the proposals outside their role, and only for government purposes. Any US government personnel who might need sight of proposals e.g. for assessment purposes, will be covered by an appropriate confidentiality agreement.
Q: Are there any specific points that international proposers need to be aware of?
A: From a technology point of view, no. We are interested in the best technology no matter what country it comes from. DASA offers the opportunity to work with worldwide suppliers and DASA has funded a number of overseas projects to date. However, for US suppliers it would be worth checking to see if their technical deliverables would be subject to ITAR. For other non-UK bidders, it would be worth checking to see if their technical deliverables would be subject to any export controls; please see Section 11. Export Control.
19.2 Scope Questions
Q: Can you provide examples of technology that you are using right now that you want to replace?
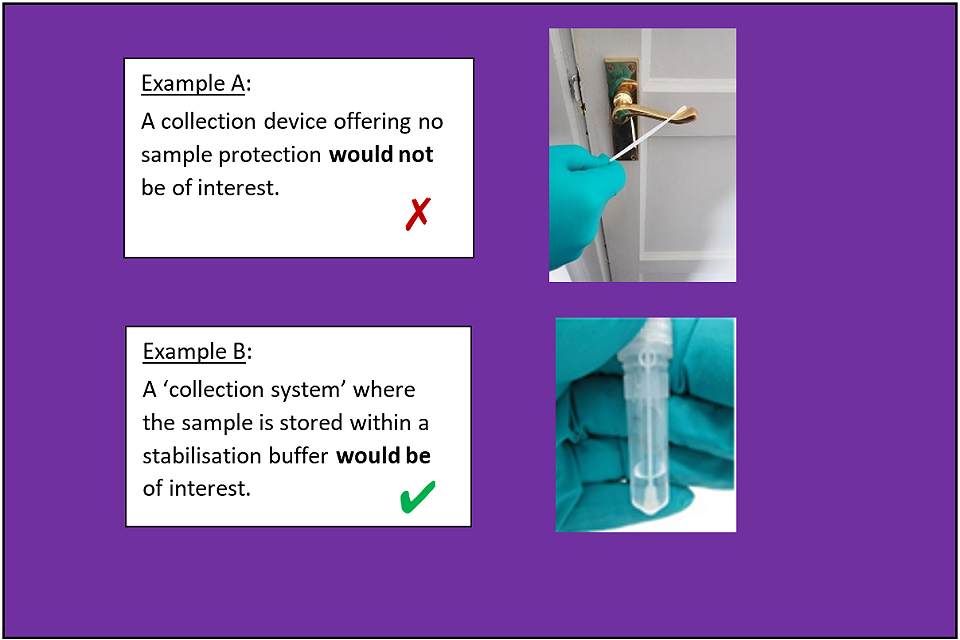
A: A simple swab is not good enough; it might collect a sample but it will not protect it (see ‘Challenge 2: Collection Systems – an example scenario ’, below).

Example scenario for Challenge 2: Collection Systems
From the screening side, something very specific like a lateral flow assay is very useful for specific tests, but we are more interested in general material properties; carrying many lateral flow devices is not practical (see ‘Challenge 1: Screening Tools – an example scenario’, below).

Example scenario for Challenge 1: Screening Tools
Q: You mentioned swab taking is outdated - have you any other collection methods you currently use to avoid this?
A: We have tried a number of different approaches instead of swabs to see what is best; it is a challenging problem. We would like to be guided by suppliers and see evidence for a suitable alternative.
Q: With respect to collecting biological samples, are you aiming to preserve them (e.g. maintaining sample integrity / cell viability) or seeking the ability to further characterise them (e.g. by preserving viral expression)?
A: Expression patterns are of interest, although we are not sure whether it is possible to preserve materials in this way at this time and it is also unclear how valuable the information would be from an analytical perspective Nevertheless if it is possible it would be an interesting avenue to explore. Overall, the better we can preserve a sample the more chance we have of analysing and attributing the hazardous ChemBio material (i.e. the more tools and approaches that can be used) (see ‘Challenge 3: Forensic Exploitation – an example scenario’, below ).
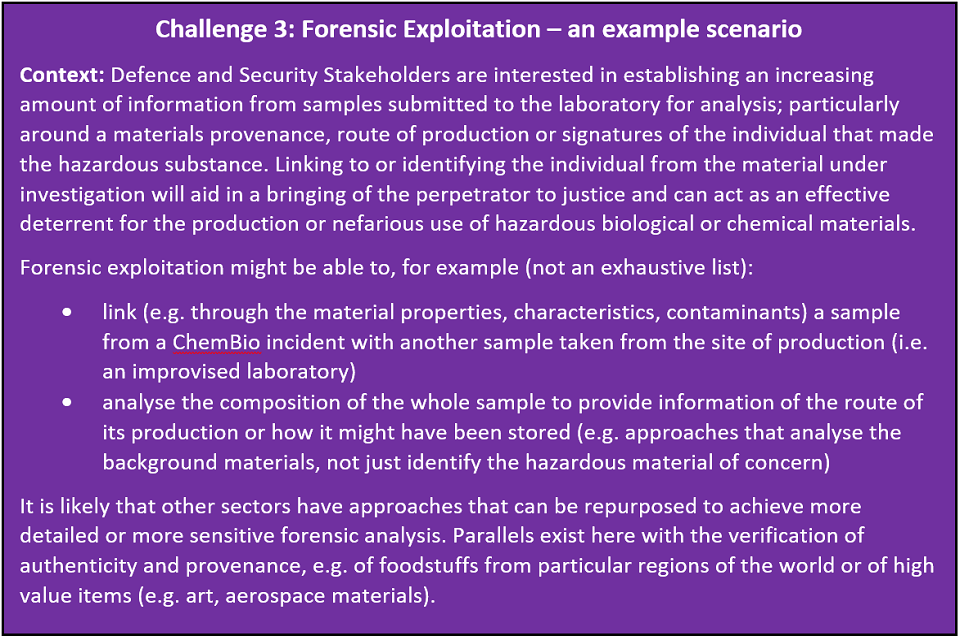
Example scenario for Challenge 3: Forensic Exploitation
Q: Do we have any examples of previous expenditure or market analysis for biological sample storage? Is this available through DASA?
A: This is not necessary for this competition. The information in the competition document is what is available for suppliers to work from.
Q: Could you give some examples of what you mean by classification as opposed to identification, with respect to the screening challenge?
A: Classification is determining whether it is a chemical or a biological hazard; we are seeking technology that can distinguish between these two classes (see also ‘Challenge 1: Screening Tools – an example scenario’, above). Once that is established we can look in more detail, e.g. at the biological properties of the material such as enzymatic activity or ion channel activity within a cell. We are interested in understanding the class of biological activity, e.g. neurological activity or ion channel inhibitory properties, therefore more about the character of the material rather than simply stating ‘it is agent X’. Traditional methods are usually highly specific for a range of agents by matching to a library; whist these are great for well-established hazardous ChemBio materials – a sample of ‘unknown’ contents containing a hazard that might not be present in the library would therefore not be detected as a consequence. Hence we are looking for potential new screening technologies that can provide information on the properties of as broad range of materials as possible rather than identification of a small range of hazards.
Q: Are you primarily interested in blood and urine and other biological samples?
A: Anything of a biomedical nature is of interest to us (e.g. including hair, skin) as well as blood and urine. Other biological substances of interest are listed in table 1 of the completion document (e.g. bacteria, virus, proteins).
Q: Would the screening of fluids be of interest?
A: Yes – the screening of fluids is of interest here as well as solids. We do not always know the nature / state of the samples that we might receive for analysis; therefore as broad a capability as possible is the ideal. Please refer to Table 1 for a summary of the technical requirements that form the basis of the competition.
Q: Would technology that can only analyse fluids be of interest?
A: No.
Q: Are you interested in methods for collecting airborne material?
A: We are primarily interested in the collection of samples from surfaces. However, other parts of defence are interested in methods of collecting airborne material; we suggest you contact your DASA Innovation Partner if this is relevant to you.
Q: Are you looking for stand-off solutions for screening or are close-in collection approaches also acceptable for the screening stage?
A: Close-in is more our domain. We are expecting people to get close up to the materials when collecting and screening samples whether they be deployed on Operations or screening a sample within an analytical laboratory setting.
Q: Is collection using a remotely-piloted / robotic device acceptable?
A: We are interested in stabilising the sample; how it’s collected is less of an issue and driver for this competition. Maintaining the sample and getting it back to the field lab or the established lab intact is more important; we want to be able to perform a detailed analysis on the sample in its best state possible.
Q: Hand held analysers (using Raman IR) are already available for counterfeit (oil) analysis. They are sample tested and analysed on site. Have you explored this?
A: This is not something we have looked into in detail for this area specifically, although it is possible other parts of Dstl have undertaken an evaluation of this type. There are a number of commercial-off-the-shelf IR-based technologies that are used by the first responder communities at present.
Q: What are your thoughts on using battery packs as an alternative to mains electricity for screening technologies used in austere environments?
A: Battery packs can have a utility in supporting technology in austere environments, but they can also create challenges such as additional weight to carry and the need to recharge them when in the field. On this basis we try to avoid using them if possible. If they are sustainable and light battery packs that can be carried easily that would be more attractive, but the aspiration is to reduce their overall use.
20. Clarification Questions
20.1 General Questions
Q: If planning a collaboration, how will the project get the relevant funding to each site and what will the mechanism of the funding be?
A: One individual submits the application and the other is named as a collaborator. If funded, a contract is made between DASA and the primary applicant. That organisation is responsible for paying the other and dealing with any intellectual property questions / issues.
Q: Is there a desire for suppliers to engage with industry?
A: This is not an expectation or a requirement. You can build a team that includes industrial partners, and that often leads to a richer proposal. Multi-disciplinary teams can allow you to go further, but this is not a requirement or a determinant of funding.
Q: At what stage is it expected that suppliers identify the end-user; at application stage, or as part of the project?
A: We do not anticipate that you have these contacts already. Some suppliers might have and if you have a useful collaboration that is fine, but it is not a requirement.
Q: Can suppliers access subject matter experts, e.g. at Dstl, for their input to the proposal?
A: No, Dstl technical experts cannot aid the development of the bid, it would give a commercial advantage. However, if funded, the supplier would gain a technical partner (TP). Specific questions relating to the appropriateness of a proposal can be submitted via DASA at accelerator@dasa.gov.uk.
Q: Is match funding desirable? Or funding leveraged elsewhere as a result of the project?
A: We do not require match funding and it offers no benefits - proposals are assessed on their own merits. Sometimes the match funding can relate to work carried out elsewhere, however the DASA project is viewed as a standalone project and assessed on its own merit. Expect to deliver what you state for the money you request, that’s the most important thing.
Q: What is the role of the DASA Innovation Partner?
A: DASA has an outreach team, the innovation partners who are based all across the UK, including one for international suppliers. Whoever the applicant is, is advised to reach out to your DASA Innovation Partner to guide them through the application process.
Q: Can we put in more than one application?
A: Yes, more than one proposal is fine from DASA perspective. Make sure they are independent and do not rely on each other; they might not both get funded. You will find it helpful to contact your regional DASA Innovation Partner for advice.
Q: We have applied to DASA before and not been successful. Are we allowed to resubmit to this call?
A: If you were invited to resubmit a proposal, this will be stated in the feedback you received. We do not accept resubmissions of exactly the same proposal if it was not previously funded. However, you can apply again for another competition. The important thing is to address the problem outlined in the competition document. It would probably be helpful to speak to your regional innovation partner, who can help support you with a new proposal. Please refer to DASA’s guidance on resubmission.
Q: As a Dstl supplier / previous supplier, are we eligible to apply?
A: Being a supplier / previous supplier is not an issue; you can still apply.
Q: Could you clarify how the funding will work between US and UK?
A: The US and UK have both contributed to the available funds and there will be a joint decision about funding for the competition. Only one application is required and all the information is in the competition document.
20.2 Scope Questions
Q: Would screening waste water be in scope?
A: Not specifically waste water for this competition. We suggest a conversation with your regional DASA Innovation Partner, who could advise whether other themed competitions or the open call might be better options for this approach. However, screening tools that can be repurposed to analyse for a broad range of hazardous materials (e.g. biological, chemical etc) within liquids are of interest including, from fresh water.
Q: Are you only interested in solid and liquid, or are gases applicable too?
A: Generally, samples laid on surfaces is what we are interested in. We want better ways of collecting and screening those. Solids and liquids are both in scope. Substances in the air (i.e. aerosol extraction) are of interest to other parts of the organisation but this is not part of this competition. Please refer to Table 1 for a summary of the technical requirements that form the basis of the competition
Q: Is chlorate, as a component of chlorate-dependent explosives, something you are interested in?
A: Explosives are not part of this call. The focus is on chemical and biological hazardous substances. Please refer to Table 1 for a summary of the technical requirements that form the basis of the competition.
Q: What chemicals are priority?
A: There is no priority list for the type of chemical we would like to detect. We want to detect as broad a list of chemicals as possible. In an ideal world, new screening tools or approaches would not simply indicate the presence of established hazardous chemicals (e.g. through library-based approaches) but also include a wider range of chemical materials in order to assess the likely composition of a sample (see ‘Challenge 1: Screening Tools – an example scenario’).
Q: What type of biological sample are you interested in: bacterial, viral, fungal?
A: For both the screening and the collection challenge, the answer is as broad a range as possible, including protein, non-protein toxin and clinical samples (e.g. blood, urine).
Q: Are miniaturisation and automation attractive features for a device?
A: Miniaturised equipment is useful in the lab (i.e. as screening will take place in containment cabinets; see below) as well as in the field where minimising the burden on deployed first responders is key (e.g. minimising weight, battery / power requirements).
Q: Are you looking for things to use in a lab or in situ?
A: The UK has more of a focus on lab-based screening tools and the US has more of an interest in deployable technologies. Nevertheless, the technical challenge remains the same with respect to developing a screening tool that is small, robust, and reliable and that can screen for as broad a range of biological and chemical hazards as possible.
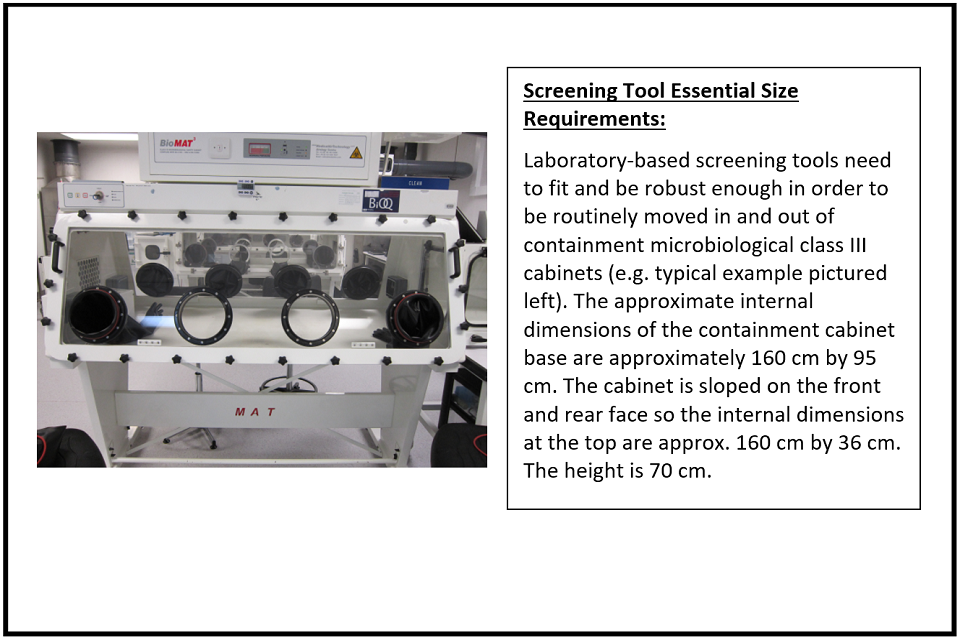
Q: What size of device will be useful?
A: Whether to be used in the field or as a lab-based screening tool, the system is required to be small and robust. Lab-based systems will obviously have access to power but must be able to fit within a standard microbiological safety cabinet. These have portholes and there is a limit to the size and dimension that can be accommodated inside (see picture and text inset below for specifications; see also ‘Challenge 1: Screening Tools – an example scenario’, above).
Q: Can a supplier submit something that is commercially available off the shelf (COTS) but which needs to be repurposed for a new use?
A: Yes, it is not an issue if the solution is COTS – it is more likely to be a plus point because it could mean a quicker route to market. You would need to demonstrate its use for the intended application. Transferring a technology into a new area such as the challenges outlined in the ’Catch the ChemBio SCENT!’ competition document means the approach might be able to move through TRL more quickly.
Q: Is testing and trialling of COTS equipment of interest?
A: This would not be suitable for DASA funding. DASA funds innovative projects that are currently low-to-mid TRL to develop them further. You can find out more about our aims and the type of projects we fund through the DASA website or you may find it useful to have a conversation with your regional DASA Innovation Partner.
Q: Is it acceptable to build and test a prototype?
A: In principle, yes, however you would need evidence that it might work for the relevant application.
Q: Do we need to know specific examples of baseline technology used in the field in order to make comparisons with the state-of-the-art, or should we compare to something else?
A: If there is no obvious market leader, stress the breadth of molecules your device can assess and what advantages it has, e.g. smaller, faster, wider scope etc. Further, for the collection system, bench-marking against COTS systems that offer no form of protection to the sample would be one option for suppliers to consider.
20.3 Challenge 1: Screening Tools
Q: From the competition document, what is meant by an unknown sample?
A: Something that has been seen before rather than it being new kind of threat; the type of substance is known to exist (i.e. whether it is a chemical or biological threat). From a technical perspective, current screening tools can be limited by the database or libraries that are held within the instrument meaning they can only detect or identify ’known’ materials (i.e. hazardous materials that are not in the library will not be picked up by these screening tools as a consequence).
Q: Are you looking for something that can screen a spectrum of substances within one sample, or to take many samples and look at each one to identify one particular substance?
A: Multiple, as long as it’s rapid. The aim is to broaden the range of materials that can be screened for and make it as wide a possible using a new innovative ways of screening samples.
Q: What chemicals are you interested in identifying?
A: In an open marketplace, we will be looking for suppliers to use analogues of exemplar substances. There is no need to use hazardous substances at this stage.
Q: Is the aim to identify whether a substance is chemical or biological, and once that’s known, to start narrowing it down from there?
A: Yes. It would be good if the identification goes across chemical and biological substances, but we appreciate that is a big ask. We are happy with something that addresses one or the other and the more information it can give, the better.
Q: Are you looking for new methods or technologies (kit) that will aid screening?
A: Yes, we are looking for new ways of approaching screening (broadening the scope of the materials that can be detected or identified and limiting reliance on library identification of a small subset of materials). If the method that is developed ends up in a kit, that’s great, but we are not anticipating that from this phase of the competition, which is seeking proof of principle only. Being able to resolve / triage between chemical or biological hazards when screening a sample would be a good start would be one example for a start (see ‘Challenge 1: Screening Tools – an example scenario’).
20.4 Challenge 2: Collection Systems
Q: Is the retention of sample sufficient or do you need to stop chemical changes in the sample?
A: Ideally, we would like to prevent any chemistry in the sample before analysis.
Q: What type of collection are you looking for?
A: Portable (hand held) surface collection systems / devices are of interest that can be operated easily within personal protective equipment. This could be pre- or post-event but needs to offer improved collection by stabilising the material for extended periods of time and without cold chain storage (see ‘Challenge 2: Collection Systems – an example scenario’). Aerosol collection is not of interest within this competition.
Q: What storage improvement are you looking for?
A: We are interested in getting the sample that has been collected back to the lab as intact as possible (e.g. preserving the precursors, breakdown products, viability of the agent).
Q: What scenarios are of interest?
A: We want to improve on current sampling techniques by developing a new collection system that will collect samples from a surface and stabilise the whole contents of that sample, preserving it for safe transport back to the laboratory for analysis and testing (see ‘Challenge 2: Collection Systems – an example scenario’). It is accepted that separate collection systems may be required to be developed for either biological, chemical or biomedical samples.
Q: What is the most difficult aspect of swab testing?
A: Fundamentally, swabs do not offer any direct form of protection to the sample and therefore do not alone represent a type of collection system desired for this competition (see ‘Challenge 2: Collection Systems – an example scenario’).
Swabs are the standard collection tool and can work well for the removal of materials from surface, however, they do not stabilise material in any way. In addition, whether it is a biological or chemical material, the swab might require cold chain storage, which can be impractical in austere environments. Also, stabilising / blocking any ongoing reactions within the materials would be a key requirement that isn’t offered by current technologies. Finally, the composition of the swab head can also affect extraction efficiency (i.e. meaning it might pick up the sample from the surface effectively but the materials may not easily be removed / collected back from it. These are all areas where improvement would be useful.
Q: Why do you want to stabilise - for shelf life, viability, functionality?
A: All of the above. We want to maintain the stability and viability of biological samples so we can maximise the analytical value of that sample. For example, by keeping bacteria and viruses alive, we can exploit these samples in much greater detail.
Q: What biological and chemical tests do you perform on the samples ?
A: Many techniques, including but not limited to PCR, microscopy. We want to be able to culture back from the swabs, (e.g. viruses into cell lines or bacterial culture). For chemical materials, analysis can utilise a number of different mass spectrometry techniques (e.g. GC-MS, LC-MS, ICP-MS).
Q: What material is good for a swab to be used for microscopy?
A: A collection system that preserves the integrity of the material is the key point here, rather than the optimal tool for one particular analytical technique.
Q: Is swabbing the environment the same as swabbing from patients?
A: In some ways, as the tools may be broadly similar in their physical appearance or composition. Currently, we have to respond to whatever has been passed to us after collection. However, through work undertaken during ‘Catch the ChemBio SCENT!’ we hope to take a more evidence-based approach where a specific collection tool will be determined / optimised for an intended application (e.g. for the collection of biological or chemical or biomedical samples)..
Q: Is there a difference in the way you would collect samples depending on the environment, (e.g. harsh environments such as extreme temperature, increased danger)?
A: No. We want to be in an informed position to demonstrate an effective way of collecting specific substances or groups of substances in different environments. Across the Defence and Security first responder community, at present, there is no single standard practise or tool recommended for the collection of hazardous chemical and biological materials We are looking to establish this by undertaking this competition.
21. Catch the ChemBio SCENT! Webinar
Catch the ChemBio SCENT! Webinar
